Spatial and temporal control over chemical and biological processes plays a key role in life. Excitation with light is an especially elegant mechanism of interaction with molecules and abundant examples are known in nature for light-induced control. In Frankfurt, we have methods for using light as “mild reagent” in Chemistry and Biology with very high temporal (down to 10 fs), spatial (down to 50 nm) and absorption selective control as well as expertise in the synthesis of light-responsive compounds and in the theoretical description of light-molecule interactions.
There are two fundamental ways of controlling processes with light: A light stimulus can either irreversibly “trigger” a process or light with two different wavelengths can induce a reversible transition between two states (for example ON and OFF). The latter can be realized either using synthesized photoswitches or (modified) natural systems. The first method is usually referred to as “uncaging” while the second method is called photoswitching with the emerging field of optogenetics, if the photoswitchable systems can be expressed using cellular machineries. Prominent examples for synthesized photoswitches are azobenzene derivatives while probably the most frequently used work horse in optogenetics are channelrhodopsins. A full discussion of the principles and an account of the developments in the recent years are far beyond the scope of this introduction. Hence, we refer to a published review article.
Reversible switching of activity is not always necessary. In the irreversible uncaging approach, it is generally much easier and more predictable to obtain a “binary” ON/OFF control: If one can identify a key site in an active molecule – a reactive functional group or an important interaction site – which is responsible for its activity, one can try to block this position covalently with a photolabile group. If the photocleavage process can be optimized to occur without side reaction after irradiation, the unmodified active molecule itself is recovered with its full activity. However, uncaging is inherently irreversible and at least in the traditional way of implementation it allows only triggering one single event.
This RTG focuses on the uncaging approach to light-control. Even though the first examples of the uncaging principle were published already in 1977, this field has recently moved in entirely new directions – also due to the scientific activity of the PIs involved in this RTG application. For example, caged nucleic acids were almost unknown until the work of Heckel, Schwalbe and Fürtig. The Tampé group has done pioneering work on the in situ assembly of protein complexes by triggering high-affinity interactions over a KD-ratio of six orders of magnitude with light.
The PhD theses of this Research Training Group depend on each other. Each of them requires a certain core expertise but also at least two neighboring expertises for its success.
The goal of this Research Training Group is to push the caging technology to new limits by
‐ developing new irradiation strategies and new caging groups for (wavelength-) selective one- and two-photon uncaging and by
‐ exploring new applications for complex scenarios of light-control
Despite the long history of photolabile (protecting) groups, wavelength-selective approaches are only just becoming available and the number of publications on this subject is growing steadily. Likewise, the problems of two-photon uncaging are far from being solved and obtaining caging groups with high two-photon action cross section is still a significant problem. In these two fields, we believe that important progress will happen in the near future.
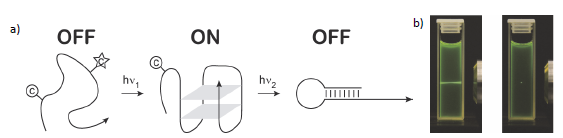
In proteins, peptides and nucleic acids with caging groups that could be addressed by using different wavelengths individual caging groups could be photocleaved in subsequent steps (Figure 1a). Therefore, considerably more complex light-driven scenarios (reactions, conformational transitions, etc.) e.g. folding involving one or multiple folding intermediates, could be initiated – keeping all the advantages of the uncaging approach (vide supra). Two-photon uncaging is based on the concept of two-photon absorption and would be another concept to achieve more complex scenarios: It uniquely allows irradiating volumes of 1 femtoliter (1 μm³) in 3D or less with high spatial resolution (Figure 1b). Designing these properties is far from being trivial and can only be performed in a tight interaction between sophisticated theoretical prediction, synthesis and ultrafast optical spectroscopy – where this RTG can draw on existing productive networks.
Apart from the development of new uncaging strategies this RTG explores new complex scenarios of light-control with special foci on NMR spectroscopy and advanced light microscopy as methods (vide infra). In a number of these applications the newly developed complex strategies of light-control will be put to the test.
The added value of performing this research endeavor in the format of an RTG lies in the fact that the range of required expertise is considerably broad (theoretical calculations, organic synthesis, photochemistry, ultrafast optical spectroscopy, NMR spectroscopy, mass spectrometry, laser technologies, advanced microscopy techniques, protein engineering, specific applications etc.). Any single or bilateral research collaboration is likely to be stuck in their particular field of research.
For success beyond these limitations the PhD students need to be scientifically supported in a structured way. The PhD students must become a closely interacting group where one individual can understand profoundly the questions of at least two neighboring disciplines and generally the questions in the entire group. To this aim – in which the support from the side of the supervisors goes beyond the one of a Research Unit – this RTG will provide the required tight interaction and structured exchange of knowledge and results among the PhD students and with the supervisors (so that the supervisors will grow together, themselves).
A central theme of this selection of researchers is that biomolecular applications require theoretical prediction - prediction promotes synthesis, which enables spectroscopy which calibrates theory and drives applications.
